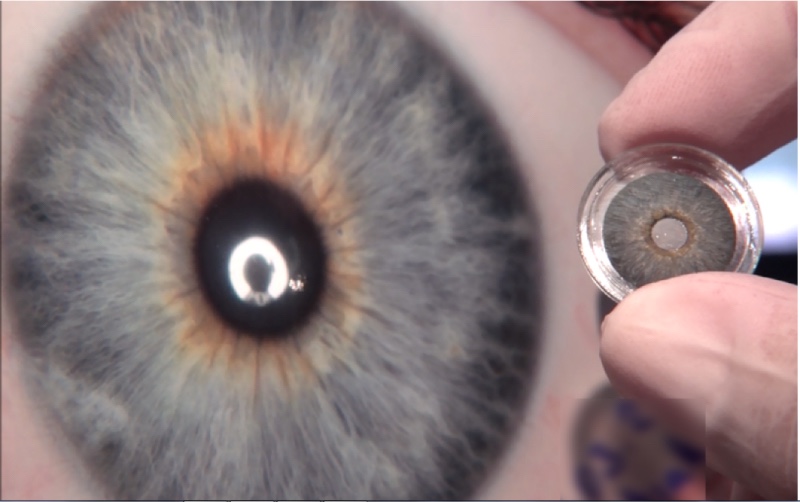
The iris prothesis CUSTOMFLEX® ARTIFICIALIRIS made by HumanOptics AG has received FDA approval. This makes it the only product of its kind currently approved in the highly attractive US market to address medical needs which until now have not been met there.
Dr. Pierre Billardon, CEO of HumanOptics AG, is delighted with the approval: “Our individually customized iris implants are unique products with exceptionally high patient benefit, which is why our ARTIFICIALIRIS was included in the FDA Breakthrough Devices Program on December 1, 2017. Just a few months later the device received FDA approval. This makes it the only product of its kind currently approved in the highly attractive US market to address medical needs which until now have not been met there.”
The ARTIFICIALIRIS will be used to treat patients who have partially or completely lost their iris due to an accident or were born without one. This condition is highly stressful for patients in their daily social lives. It is also often associated with a whole host of other medical indications such as increased sensitivity to light, vision loss or an above-average risk of glaucoma and cataracts.
HumanOptics individually models each ARTIFICIALIRIS based on the original appearance of the patient’s iris. The prosthetic consists of a flexible silicone material which is inserted into the eye using the small cut technique, making it a well-tolerated procedure. This ensures both medical and aesthetic patient reconstruction and thus achieves a very high level of satisfaction.
HumanOptics AG has already earned an outstanding reputation in the highly specialized market for eye surgery in the USA. In the context of a medical study started in 2013, more than 600 successful operations have already been performed at 12 clinics in the USA. The market potential for our product encompasses some 150 clinics and between 1,000 and 1,500 patients per year.
This has given Dr. Billardon good reason to anticipate high sales and earnings potential for this product: “We expect the artificial iris to develop into an important pillar of our company over the medium term. Many of our current surgeons already have waiting lists of patients who need our artificial iris but could not be treated in the study. In addition to the significant market potential in the USA, the FDA approval will also have a positive impact on our European markets, where we are already permitted to offer the product today.”