Monofocal premium IOL for optimal cataract treatment
The ASPIRA-aAY is a one-piece monofocal capsular bag lens with all the advantages of a premium IOL:
Aspheric, aberration-free optic design for improved contrast sensitivity
XL delivery range
MICS - for astigmatism-neutral implantations
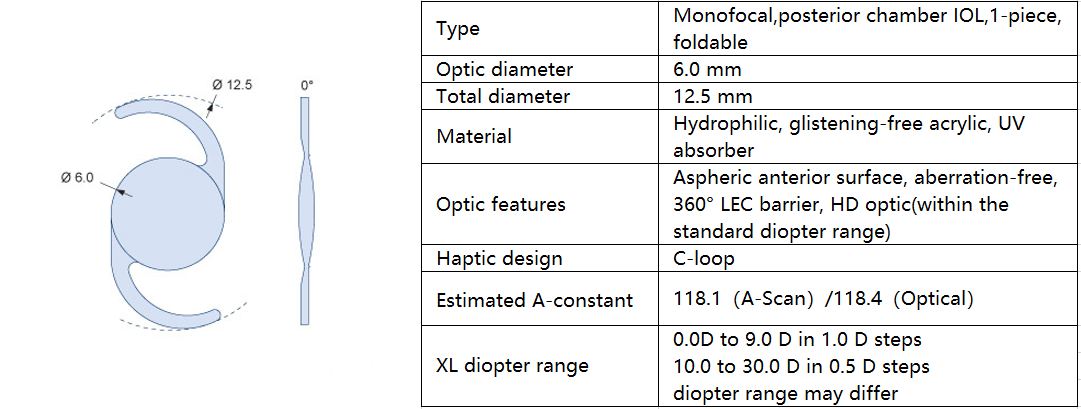
Monofocal 1P
ASPIRA-aA
Excellent imaging quality
Exceptional delivery range
Aberration-free – suitable for all patients
Spherical aberration (SA) is a higher-order imaging error that can affect the contrast vision of patients after IOL implantation. Aberration-correcting IOLs are based on average values of corneal SA measurements. However, these IOLs can only partially compensate or even amplify the patient specific SA, since it varies greatly within individuals.
The aberration-free optic of the ASPIRA-aA offers the basis for excellent imaging quality. The aspheric optic design is aberration-neutral, so that no additional imaging error is introduced into the eye. It is suitable for all patients, regardless of corneal aberration. Compared with spherical or aberration-correcting IOLs, patients benefit from increased contrast sensitivity and depth of field.
XL diopter range
The ASPIRA-aA is available in an exceptionally wide delivery range beyond standard values commonly available on the market. IOLs with a sphere of 0.0 to 30.0 D provide optimal treatment, even for patients with extreme visual defects.
Premium IOL ASPIRA-aA
The advantages of the premium IOL platform
The high-precision manufacturing technology (Sub-nano resolution technology) enables the manufacturing of optic surfaces with an accuracy in the nanometer range. An additional production step - polishing - which is often necessary with conventional IOLs and reduces the optical quality, can be omitted in our process. The result of this cutting-edge technology is a surface of premium quality for brilliant, clear, and sharp images.
360° lens epithelial cell barrier and sharp optical edges offer effective prevention of postoperative post-capsular opacification.
Flexible material properties allow minimally invasive, astigmatism-neutral implantation (MICS).
MICROCRYL® is completely glistening-free and shows excellent uveal biocompatibility.
Our premium IOLs are developed and produced exclusively in Germany according to the strictest quality specifications and thus offer the highest quality with maximum product safety.